GAS SAMPLE DATA Volume of sample 900 mL Temperature 25 C Atmospheric Pressure 745 mm Hg Equilibrium Vapor Pressure of H 2 O 25 C 238 mm Hg. As a result the pressure of the gas that has displaced the liquid water is the sum of the pressure of the gas plus the.
A 60 0 Ml Sample Of Co 2 Gas Is Collected Over Water At 70 0 And 101 3 Kpa What Is The Volume Of The Dry Gas At Stp Socratic
A 976 mL sample of hydrogen gas is collected by water displacement at 200 oC.
. If the barometric pressure is 7520 torr and the water vapor pressure is 175 torr what is the partial pressure of. The pressure of the collected gas which is the same as the atmospheric pressure in the laboratory is measured to be 7695 torr. As a gas is collected over water it becomes saturated with water vapor and the total pressure of the mixture equals the partial pressure of the gas plus the partial pressure of the water vapor.
A sample of gas collected over water at 42 C occupies a volume of one liter. The relevant data are given in the following table. Nitrogen oxygen and hydrogen can be collected by water displacement because they are only slightly soluble in water.
Hydrogen gas is collected by water displacement. What was the partial pressure of the oxygen collected. Depending on the temperature of the water in the graduated cylinder some molecules of liquid water.
When a gas is collected by water displacement the gas collected is not a pure gas but a mixture of gas plus some water vapor. The vapor pressure of water is 283 mmHg at 25C. Procedure used to collect gas pressure for water vapor present in the sample.
A hydrogen gas sample collected in experiment occupies 785 mL at 25 C. P H 2 O 175 mmHg vapor pressure of water at 200C P T P atm. A sample of a gas occupies 250 mL at 100 atm of pressure.
When this technique is used however the gas collected in the bottle contains a small but significant amount of water vapor. Gases collected by water displacement contain. What is the partial pressure of H_2 if 3078 mL of gas is collected over water from this method.
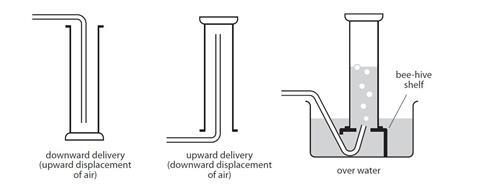
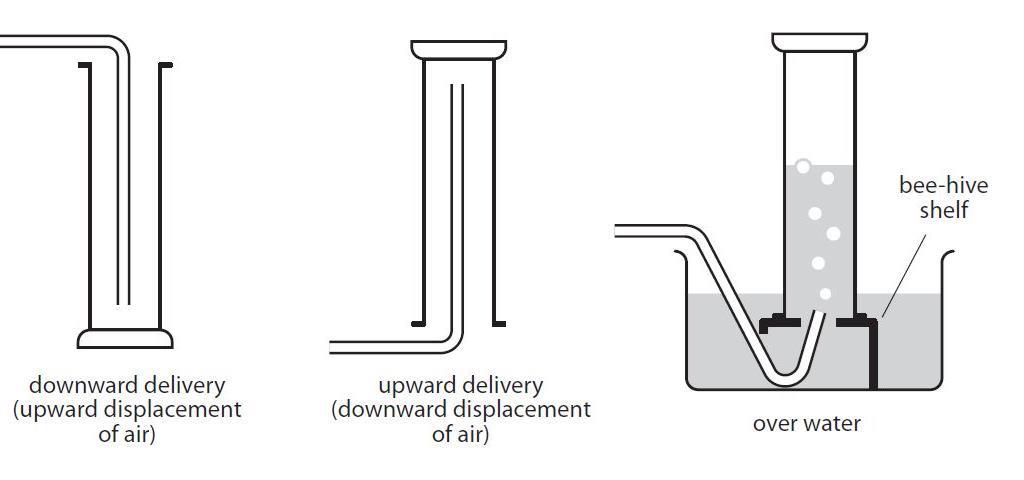
In fact any gas that does not have appreciable water solubility can be collected by downward displacement of water. When a gas is collected over water by the water displacement method it contains also water vapor wet gas. When a gas is collected over water by the water displacement method it contains also water vapor wet gas.
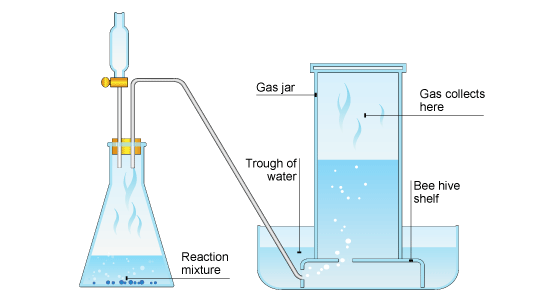
The pressure of the gas in the graduated cylinder whose volume we can directly measure is equal to the atmospheric pressure PLUS the saturated vapour pressure of water which is tabulated at various temperaturesOf course. Gases that are produced in laboratory experiments are often collected by a technique called water displacement see figure below. A student collected a sample of hydrogen gas by the displacement of water as shown by the diagram above.
The vapor pressure of water at 350 C is 42 torr. If a beaker contains oxygen and nitrogen gas what is the pressure of the oxygen gas if the total pressure in the beaker is 20 atm and the pressure of nitrogen is 15 atm. Describes how to correct gas pressure for water vapor present in the sample.
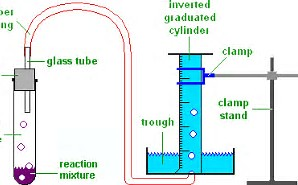
Since the nitrogen was collected via water displacement the sample is saturated with water vapor. The reaction flask is fitted with rubber tubing which is then fed under the bottle of water. Gas Collection by Water Displacement.
A sample of hydrogen gas collected by displacement of water occupied 300 mL at 24 oC and pressure 736 torr. The volume of gas collected and the gas laws can be used to calculate the number of moles of gas collected. Click Create Assignment to assign this modality to your LMS.
A 324 mL b 216 mL c 368 mL d 259 mL e 276 mL 15. During the collection the water level in the container will adjust so that the pressure inside and outside the container are. A sample of nitrogen gas was collected via water displacement.
What happens when a gas is collected over water. If the pressure increases to 200 atm while the temperature stays the same what is the new volume. How many milligrams of hydrogen does the sample contain.
For a gas to be collected by water displacement the gas should not be soluble in the water or react with the water. The vapor pressure of water at 25C is 238 mmHg. Volume of sample 105 mL Temperature 23 0C Atmospheric pressure 755 mm Hg Vapor pressure of water at 23 0C 2107 mm Hg a Calculate the partial pressure of the nitrogen gas that is collected.
Share this link with a friend. As the gas is created it will. If a gas is collected at 25C and the barometer reads 767 mmHg what is the partial pressure of the gas dry gas.
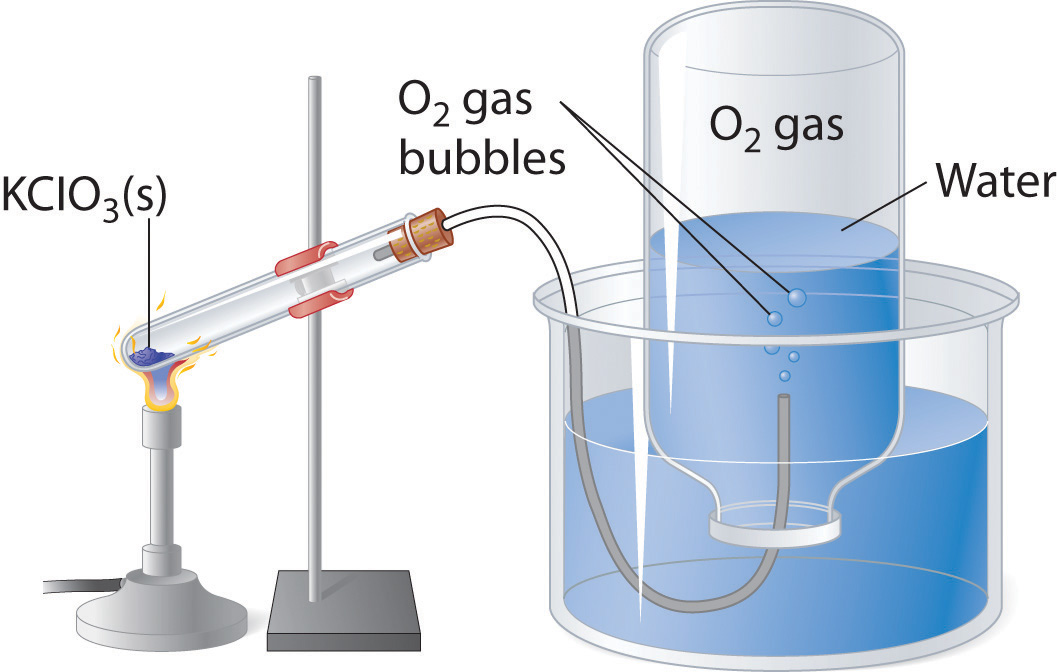
If the total pressure of the mixture at 21 C is 116 atm what is the partial pressure of. Oxygen gas from the decomposition of potassium chlorate KClO 3 was collected by water displacement. Water evaporates and there is always gaseous water water vapor above a sample of liquid water.
H2Og For a fixed amount of gas at a constant temperature the volume increases as the pressure. A gas is collected by water displacement so that its partial pressure is 5000 kPa. What volume would the hydrogen occupy if it were dry and at STP.
The tabulated value of the vapor pressure of water at 200 oC is 175 torr. A bottle is filled with water and placed upside-down in a pan of water. A 324 mL b 216 mL c 368 mL d 259 mL.
The volume of gas can be determined by the amount of water that was displaced by the gas. A sample of hydrogen gas collected by displacement of water occupied 300 mL at 24 o C on a day when the barometric pressure was 736 torr. The vapor pressure of water at 240 o C is 224 torr.
The hydrogen gas is collected by displacement of water at 350 C at a total pressure of 748 torr. As the gas is created it will displace water from the bottle. A student collected a sample of nitrogen gas by the displacement of water as shown by the diagram above.
The relevant data are given in the table below. Gas Collection by Water Displacement. What volume would the hydrogen occupy if it were dry and at STP.
But hydrogen chloride or ammonia can not be separated since both are very highly soluble in water and will react to form hydrochloric acid or ammonium hydroxide respectively so statement 1 is not true. If a gas is collected at 25C and the barometer reads 767 mmHg what is the partial pressure of the gas dry gas. The vapor pressure of water at 240 oC is 224 torr.
The barometric pressure and the temperature during the experiment were 7310 mmHg and 200C respectively.

What Gases Can Be Collected By Water Displacement Socratic

Physical Characteristics Of Gases Ppt Download
Generating Collecting And Testing Gases Experiment Rsc Education

Gas Laws Ppt Video Online Download

Gas Collection By Water Displacement Ck 12 Foundation
Generating Collecting And Testing Gases Experiment Rsc Education
0 comments
Post a Comment